Introduction: Systemic light chain (AL) amyloidosis is a plasma cell dyscrasia in which a toxic plasma cell clone causes devastating multiorgan complications and death, and substantially affects the quality of life of patients (pts). Current therapeutic options are not regulatory approved and are typically based on modified treatments that are used for multiple myeloma. The aim of EMN23 study is to thoroughly describe patterns of AL amyloidosis management in a large-scale real-world evidence (RWE) setting throughout Europe, to understand different treatment strategies and their evolution, and evaluate resource utilization.
Methods: EMN23 is an ongoing, retrospective, observational, multicenter study aiming to enroll 5000 pts in 10 countries (13 Sites) across Europe; Austria, Czech Republic, France, Germany, Greece, Italy, the Netherlands, Portugal, Spain, and UK. Pts must have documented systemic AL amyloidosis, and a first-line treatment start date between 2004 and 2018. Results are presented in 2 chronological cohorts, 2004-2010, and 2011-2018, with 2010 being an important landmark, since bortezomib, cyclophosphamide, dexamethasone (CyBorD) regimen was introduced in clinical practice. Pts who received treatment in the context of an interventional clinical trial were not analyzed for treatment and efficacy results. The current analysis presents baseline disease characteristics, treatment patterns and efficacy outcomes. Informed consent or a non-opposition letter was collected for all alive pts, depending on the regulatory environment of each country, and a waiver of consent was obtained for the deceased.
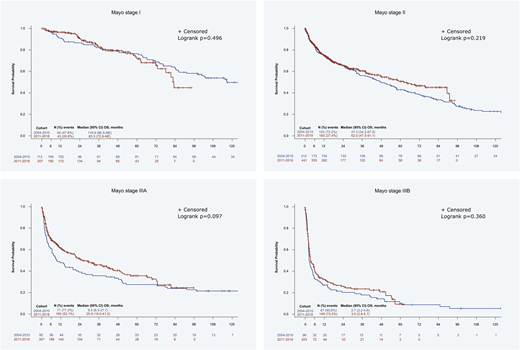
Results: In total, 2031 pts from Austria (1.9%), Czech Republic (0.9%), France (9.2%), Germany (3.6%), Greece (12.6%), Italy (58.7%), the Netherlands (7.2%), Portugal (1.0%), and Spain (4.9%) have been analyzed; 67.9% of pts started treatment after 2010. Median age at diagnosis was 66 years and 54.5% were males, while 48.2% had ECOG performance status ≤1. Heart (71.9%), kidney (67.2%), soft tissue (19.7%) and nervous system (18.5%) were most often involved at diagnosis, and 17.2%, 36.2%, 21.1% and 15.2% of pts were at cardiac stage I, II, IIIA and IIIB, according to Mayo staging system, respectively, while for 10.3% of pts staging was not reported. There was a slight increase of pts with stage III in the more recent era. In the period 2004-2010, 52.8% of the pts had at least 2 lines of therapy, which dropped to 35.2% in the period 2011-2018. A 6.4% of pts received autologous stem cell transplant at first line. The prevailing first-line regimen in 2004-2010 was melphalan with dexamethasone (MDex; 50.6% of the pts), replaced by CyBorD (46.1%) in the post-2010 period. The predominant first-line regimens were different in various countries in 2004-2010, but in general CyBorD was the most extensively used regimen after 2010. Overall, the most frequent second-line regimen was BorD (29.8%), which was also the treatment of choice for 15.7% of the pts who had frontline therapy with MDex in the pre-2010 period; and lenalidomide dexamethasone (RD, 25.5%) which was also favored following frontline treatment with CyBorD for 10.6% of the pts in the post-2010 period. In the 2004-2010 era, 31.7% of the pts achieved a hematologic VGPR or CR at first line, which was slightly improved to 37.2% in the post-2010 period. Notably, 29.9% and 23% of pts did not achieve a hematologic response (no response or disease progression) in the two periods, respectively (p=0.001). Mortality in the first months of treatment remained roughly at the same level, with the 3-month overall survival (OS) rate at 82.3% for both cohorts, whereas the median OS increased from 43.5 months in the pre-2010 period to 50.1 months in the post-2010 period (p=0.525). There was a trend towards an improvement in the median OS of stage IIIA pts from 9.5 to 25.9 months, pre- and post-2010, respectively, however, no change was observed for stage IIIB pts (2.7 to 3.5 months).
Conclusions: Therapeutic options for AL amyloidosis have changed considerably over time. After 2010 CyBorD became the prominent first-line treatment; the proportion of pts not achieving a response was reduced by 23% and a trend towards improvement of OS for stage IIIA disease was observed, but the prognosis of stage IIIB pts remained dismal and early mortality remained unchanged. Early diagnosis is still an unmet need, and improved therapies are essential.
Palladini:Celgene: Other: Travel support; Jannsen Cilag: Honoraria, Other. Schönland:Janssen, Prothena, Takeda: Honoraria, Other: travel support to meetings, Research Funding. Milani:Celgene: Other: Travel support; Pfizer: Other: Speaker honoraria; Janssen: Other: Speaker honoraria. Jaccard:Janssen: Consultancy, Honoraria, Other: A.J. has served in a consulting or advisory role for Janssen and has received honoraria from, received research funding from, and had travel, accommodations, or other expenses paid for or reimbursed by Janssen., Research Funding; Celgene: Honoraria, Other: A.J. has served in a consulting or advisory role for Janssen and has received honoraria from, received research funding from, and had travel, accommodations, or other expenses paid for or reimbursed by Celgene., Research Funding. Bridoux:Celgene: Honoraria; Baxter: Consultancy; Janssen: Honoraria. Cibeira:Amgen: Honoraria, Other: Educational lectures; Celgene: Honoraria, Other: Educational lectures; Akcea Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Educational lectures. Agis:Janssen: Honoraria, Research Funding; Amgen: Honoraria; BMS: Honoraria; Celgene: Honoraria; Takeda: Honoraria. Minnema:Amgen: Honoraria; Servier: Honoraria; Janssen Cilag: Honoraria; Celgene Corporation: Honoraria, Research Funding; Gilead: Honoraria. Bergantim:AMGEN, Celgene, Janssen, Takeda: Membership on an entity's Board of Directors or advisory committees, Other: Speaker honoraria; Celgene, AMGEN/SPH/APCL: Other: Grants, Research Funding. Hajek:Oncopeptides: Consultancy; Janssen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; PharmaMar: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria. João:Takeda: Consultancy, Research Funding; Amgen: Consultancy; Janssen: Consultancy; BMS: Consultancy. Wechalekar:Celgene: Honoraria; Janssen: Honoraria, Other: Advisory; Caelum: Other: Advisory; Takeda: Honoraria, Other: Travel. Sonneveld:Karyopharm: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy; Skyline Dx: Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding. Kastritis:Amgen: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Genesis Pharma: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria.
This is a RWE study on AL amyloidosis, and currently there are no regulatory approved therapeutic options for the disease.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal